In Augmented Reality News
July 20, 2023 – MediView XR, Inc., a clinical augmented reality (AR) med-tech company, has today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its XR90 augmented reality-based surgical visualization and navigation platform.
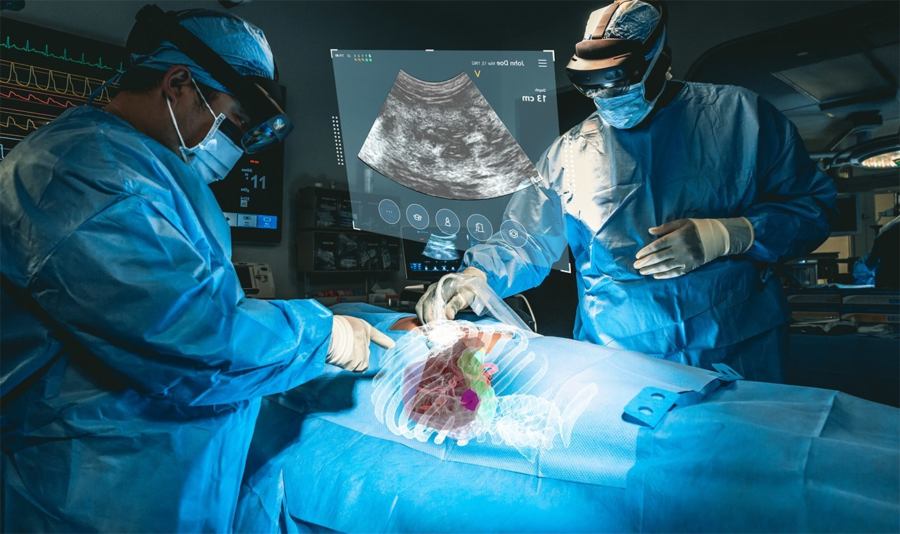
MediView utilizes AR to address some of the limitations of current medical imaging technologies associated with flat panel monitors, which limit practitioners to 2D imaging and require them to look away from a patient’s procedural site. The company’s XR90 device is intended to be used adjunctively to standard of care imaging for minimally invasive ultrasound and CT guided needle-based procedures for soft tissue and bone.
The device projects 3D virtual models of a patient’s own anatomy based on their CT imaging and combines that CT with live ultrasound to perform minimally invasive procedures, such as biopsies and tumor ablations (the use of heat or cold to kill cancerous tumors).
XR90’s augmented reality capabilities include a Holographic Light Ray that tracks and displays the path of the physician’s instrument, CT-based 3D holographic anatomy display, and live ultrasound that is projected and displayed anatomically into the patient as the clinician scans, similar to a flashlight beam.
Through Microsoft’s HoloLens 2 AR headset, clinicians are able to visualize the patient’s ultrasound, as well as displays of other procedural information to facilitate their workflow. MediView stated that the XR90 overcomes the limitations of two-dimensional imaging by providing physicians with 3D “X-Ray vision” during procedures – the ability to visualize a patient’s comprehensive internal anatomy in 3D underneath their skin, including bone, tissue, organs and vasculature.
“XR90 expands the MediView portfolio of solutions available to practitioners as they look to simplify, democratize, and inform care delivery with the ultimate goal of improving and expanding access to the best care,” said Mina Fahim, President and CEO of MediView.
MediView’s technology also enables clinicians at remote locations to collaborate in real-time with shared visualization, communication, and the ability to provide guidance during procedures for collaborative patient care. These features can provide increased support for understaffed facilities, rural or underserved populations and a distinct ability to limit caregiver and patient exposure to COVID-19 while maintaining care levels, according to MediView.
Commenting on the announcement, Adam Cargill, Director of Quality, Regulatory and Clinical Affairs at MediView, said: “This is not only the first 510(k) clearance for MediView, but it is the first 510(k) clearance for an augmented reality device utilizing live imaging combined with 3D XR visualization for pre- and intra-operative indications for use, which sets the stage for further advancements in augmented reality in the healthcare space.”
MediView recently announced that it secured a USD $15 million strategic funding round to expand its AR surgical navigation platform. To learn more about MediView’s augmented reality solutions for medical imaging, please visit the company’s website.
Image credit: MediView
About the author
Sam is the Founder and Managing Editor of Auganix. With a background in research and report writing, he has been covering XR industry news for the past seven years.
